
BMBF Consortium: Ferroptosis as a common underlying pathomechanism in tissue ischemia/reperfusion injury [FERROPath]
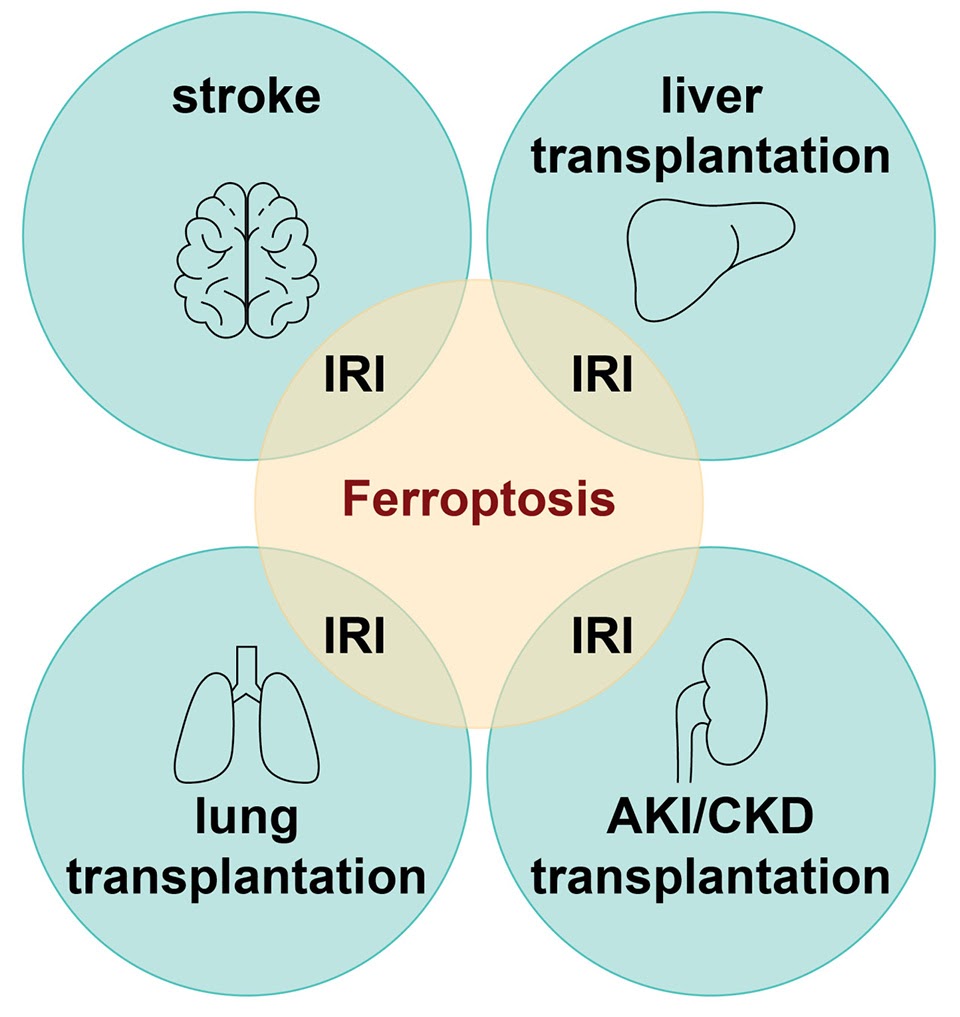
When reperfusion is induced after an ischemic event, specifically during stroke, organ transplantation, or resuscitation after a cardiac arrest, this reperfusion process can paradoxically cause additional damage to the affected tissue. Such ischemia-reperfusion injuries (IRI) are a serious complication responsible for a variety of clinically important conditions including exacerbated brain damage and acute kidney injury. Ferroptosis, a recently described form of regulated necrotic cell death, has emerged as a common underlying pathomechanism of tissue IRI, and represents a promising therapeutic target to prevent extensive IRI-driven cell loss, tissue damage, and necroinflammation. This form of cell death is characterized by iron-dependent lipid peroxidation, ultimately leading to plasma membrane rupture and death of the cell. To date, there is no biomarker available that unequivocally allows for the detection of ferroptosis, and despite the development of ferroptosis inhibitors there is no treatment for IRI.
Dr. Maria Fedorova, leader of the research group “Lipid Metabolism: Analysis and Integration” at the Center of Membrane Biochemistry and Lipid Research (ZML) at the Faculty of Medicine of the Dresden University of Technology TUD, is the project coordinator of the FERROPath consortium that will be funded by the Bundesministerium für Bildung und Forschung (BMBF) from July 2022 for 3 years. The consortium brings together six partners with highly complementary expertise in oxi-lipidomics, bioinformatics, drug discovery, immunology, organ transplantation, pre-clinical, and clinical research in a highly interdisciplinary approach based on Dresden, München, Regensburg and Essen.
Prof. Ana Casas and Prof. Kleinschnitz lead the subproject „The role of non-cell-autonomous ferroptotic cell death in acute brain ischemia“, which will analyze ferroptosis-dependent pathways in post-stroke brain damage. Here they aim to identify brain-specific biomarkers of stroke progression which could be later used as markers to assess therapeutic success and prognosis. Ultimately, the consortium strives to provide a proof-of-concept for next-generation ferroptosis inhibitors.
Publications
Bieber M, Werner RA, Tanai E, Hofmann U, Higuchi T, Schuh K, et al. Stroke-induced chronic systolic dysfunction driven by sympathetic overactivity. Ann Neurol. 2017
Casas AI, Geuss E, Kleikers PWM, Mencl S, Herrmann AM, Buendia I, et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc Natl Acad Sci 2017 Nov 14;114(46):12315–20
Bieber M, Schuhmann MK, Volz J, Kumar GJ, Vaidya JR, Nieswandt B, et al. Description of a Novel Phosphodiesterase (PDE)-3 Inhibitor Protecting Mice from Ischemic Stroke Independent from Platelet Function. Stroke. 2019
Casas AI, Hassan AA, Larsen SJ, Gomez-Rangel V, Elbatreek M, Kleikers PWM, et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc Natl Acad Sci 2019 Apr 2;116(14):7129–36.
Casas AI, Kleikers PWM, Geuss E, Langhauser F, Adler T, Busch DH, et al. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J Clin Invest 2019 Mar 18;129(4):1772–8.
Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, et al. Post-Stroke Inhibition of Induced NADPH Oxidase Type 4 Prevents Oxidative Stress and Neurodegeneration. McLeod M, editor. PLoS Biol 2010 Sep 21;8(9):e1000479. A
Göbel K, Asaridou CM, Merker M, Eichler S, Herrmann AM, Geuß E, et al. Plasma kallikrein modulates immune cell trafficking during neuroinflammation via PAR2 and bradykinin release. Proc Natl Acad Sci U S A. 2019




