
TRR332 Neutrophils: origin, fate, and function
Within the collaborative research unit TRR332, researchers from Muenster, Essen and Munich are investigating neutrophil biology. The role of intracellular neutrophil activation as well as tissue specific neutrophil production or neutrophil activation in different diseases will be studied.
Our project C06 “The role of Ly6G and CD177 as “eat me” signals to control neutrophil-mediated tissue destruction in stroke” within the TRR332 aims to unravel the molecular pathways governing neutrophil behavior, particularly their interaction with microglia, with a focus on the roles of Ly6G in mice and its human counterpart CD177 in sterile inflammation. Neutrophils are the first leukocyte subset entering inflamed tissue and are thereby crucial for responding to infectious threats Furthermore, neutrophils contribute significantly to non-infectious inflammation, such as in stroke and cancer. While their presence can aid in tissue repair, it often exacerbates conditions like stroke injury.
Our study thereby aims to identify the receptors responsible for phagocytic uptake, to investigate the kinetics of synapse formation and maturation, and to image and characterize single microglia-neutrophil pairs. In this project both in vitro and in vivo methods will be applied using primary and secondary cell culture models as well as the middle cerebral artery occlusion model in mice. Another aim is the development of strategies to overcome the lack of the Ly6G/CD177 axis, hypothesizing that their absence impairs neutrophil phagocytosis. Investigating and modulating “don’t eat me” signals post-stroke will result in developing therapeutic interventions to alleviate stroke severity, especially in Ly6G/CD177-deficient individuals.
Furthermore, we will explore the role of CD177 deficiency in human tissue inflammation through a clinical cohort study comparing participants experiencing ischemic stroke events with controls. By analyzing clinical, laboratory, and neuroimaging data, we aim to elucidate the impact of CD177 deficiency on stroke pathophysiology and recovery.
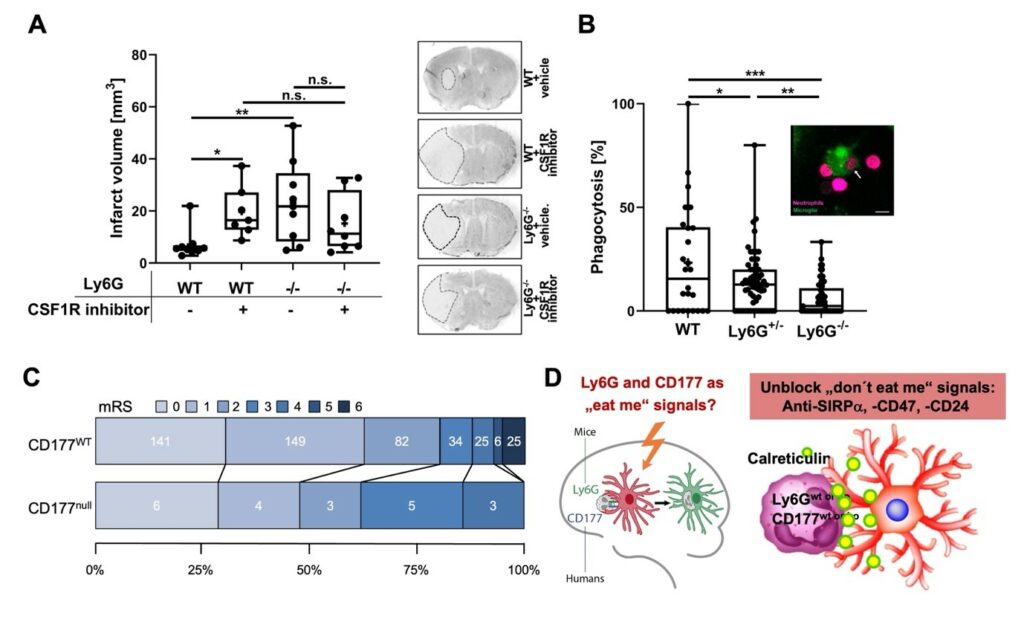
Figure 1: Ly6G or CD177 deficiency exacerbates ischemic injury in mice and human. A Transient middle cerebral artery occlusion (MCAO) was induced in mice treated with vehicle or CSF1R inhibitor 1 hour before MCAO and once daily after MCAO. Animals were sacrificed after 3 days and infarct volume was determined. While microglial deactivation by the CSF1R inhibitor increased infarct volume in WT mice, this effect was not observed in Ly6G-/- mice, which exhibited increased infarct volumes very similar to CSF1R inhibitor-treated WT mice. B Freshly prepared microglia (green) and neutrophils (magenta) were incubated for 4 hours at 37°C. The image shows a representative engulfment of a neutrophil (arrow) by microglia in vitro. Scale bar: 10 µm. The percentage of microglia which had engulfed neutrophils per view field was evaluated by confocal microscopy. C Grotta bars for functional disability assessed by modified Rankin Scale (mRS) score at one-year post-stroke in CD177 wildtype (CD177WT) and CD177 deficient (CD177null) patients in PROSCIS-B study. Numbers of patients in each mRS category are indicated in the graph. mRS = 0: no symptoms, mRS = 1: no significant disability, mRS = 2: slight disability, mRS = 3: moderate disability, mRS = 4: moderately severe disability, mRS = 5: severe disability, mRS = 6: death. D graphical summary of scientific question and project plans
Publications
Yin, D., Wang, C., Qi, Y., Wang, Y.C., Hagemann, N., Mohamud Yusuf, A., Dzyubenko, E., Kaltwasser, B., Tertel, T., Giebel, B., et al. (2023). Neural precursor cell delivery induces acute post-ischemic cerebroprotection, but fails to promote long-term stroke recovery in hyperlipidemic mice due to mechanisms that include pro-inflammatory responses associated with brain hemorrhages. J Neuroinflammation 20, 210. 10.1186/s12974-023-02894-8.
Mohamud Yusuf, A., Hagemann, N., Ludewig, P., Gunzer, M., and Hermann, D.M. (2021). Roles of Polymorphonuclear Neutrophils in Ischemic Brain Injury and Post-Ischemic Brain Remodeling. Front Immunol 12, 825572. 10.3389/fimmu.2021.825572.
Hagemann, N., Mohamud Yusuf, A., Martiny, C., Zhang, X., Kleinschnitz, C., Gunzer, M., Kolesnick, R., Gulbins, E., and Hermann, D.M. (2020). Homozygous Smpd1 deficiency aggravates brain ischemia/ reperfusion injury by mechanisms involving polymorphonuclear neutrophils, whereas heterozygous Smpd1 deficiency protects against mild focal cerebral ischemia. Basic Res Cardiol 115, 64. 10.1007/s00395-020-00823-x.





