
Network pharmacology for neurovascular diseases
Importantly, the efficacy of drug discovery is currently in constant decline. In fact, this poor translational success of biomedical research is due to false incentives, lack of reproducibility, and persistent publication bias. Additionally, the most crucial reason is our current concept of disease, i.e., mostly defined by the organ or symptom involved rather than the mechanism causing the disease. However, recent breakthroughs in genetics and bioinformatics united in network medicine aim to redefine diseases based on the underlying causal mechanism leading to a precision diagnosis and therapy. Here, the focus is primarily on the associated risk genes involved in the development of the disease. Therefore, we will ultimately treat the cause rather than the symptom.
The explosion of systems medicine opened a new concept of treatment focused on the so-called network pharmacology, which suggests that diseases are more effectively treated by two or more mechanism-based drugs, thus targeting key proteins of the same causal signalling network in a synergistic manner. Importantly, this strategy must not be confused with the common practice of combination therapy, where mechanistically unrelated drugs are co-prescribed without targeting a causal disease mechanism and therefore not acting synergistically. Instead, we here aim to restore physiological signalling for mechanism-based treatment.
Under the scope of the NEURONET scientific program, we are developing an alternative approach to understanding the disease mechanism underlying long-term post-stroke impairment and related neurovascular disorders. Following a network pharmacology approach, NEURONET aims to identify an in silico-based therapy for long-term impairment post-stroke together with potential bio- and inflammatory markers in different neurovascular indications, subsequently defining new strategies for early detection and treatment.
Publications
Nogales C, Mamdouh ZM, List M, Markus List, Christina Kiel, Ana I. Casas, Harald H.H.W. Schmidt. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci 2022; 43: 136-150. 2021/12/14.
Ana I. Casas, Hermann A.M. Mucke, Cristian Nogales, Alexandra Petraina, Antonio Cuadrado, Ana I. Rojo, Pietro Ghezzi, Vincent Jaquet, Fiona Augsburger, Francois Dufrasne, Jalal Soubhye, Soni Deshwal, Moises Di Sante, Nina Kaludercic, Fabio Di Lisa, and Harald H.H.W. Schmidt. On the clinical pharmacology of reactive oxygen species. Pharmacological reviews, 2020 Oct;72(4):801-828
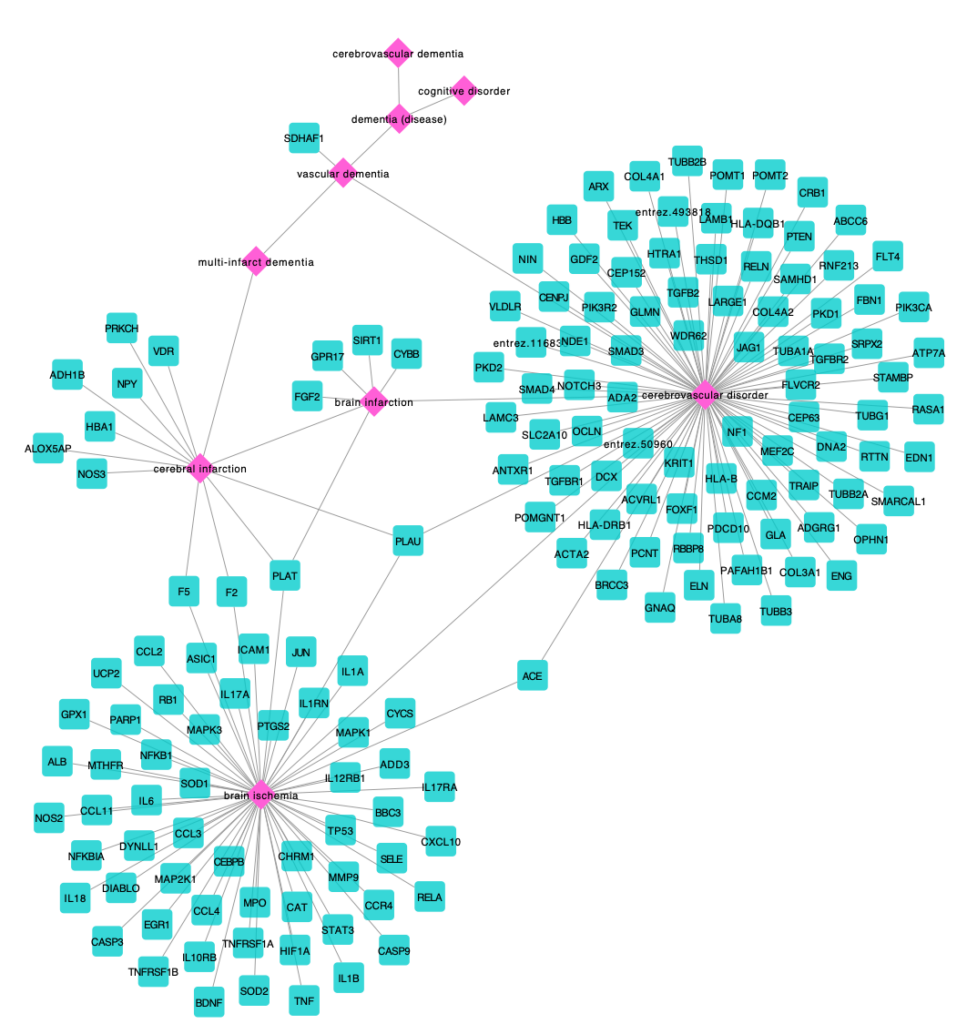
Ana I. Casas, Ahmed A. Hassan, Simon J. Larsen, Vanessa Gomez-Rangel, Mahmoud Elbatreek, Pamela W. M. Kleikers, Emre Guney, Javier Egea, Manuela G. Lopez, Jan Baumbach, Harald H.H.W. Schmidt. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. (PNAS), 2019 Apr 2;116(14):7129- 7136
Friederike Langhauser*, Ana I. Casas*, Vu-Thao-Vi Dao, Emre Guney, Jörg Menche, Eva Geuss, Pamela W.M. Kleikers, Manuela G. López, Albert-L. Barabási, Christoph Kleinschnitz & Harald H.H.W. Schmidt. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. npj (nature) | Systems Biology & Applications, 2018 Feb 5;4:8. vol.4 (1) pp. 8 *Equally contributed to this work






