
Thromboinflammation
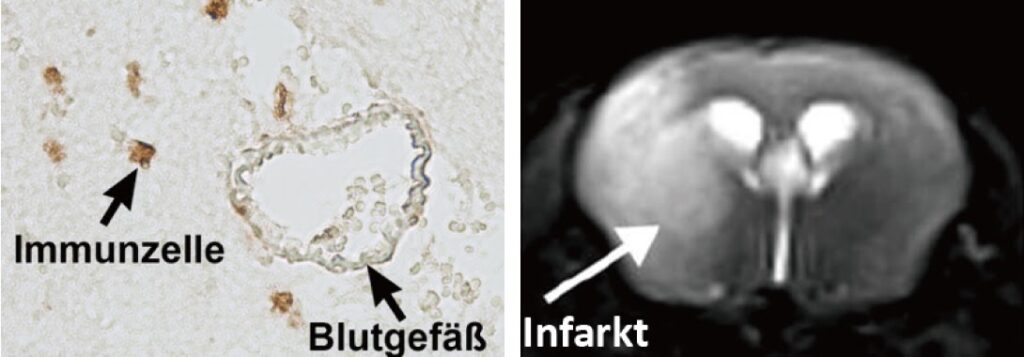
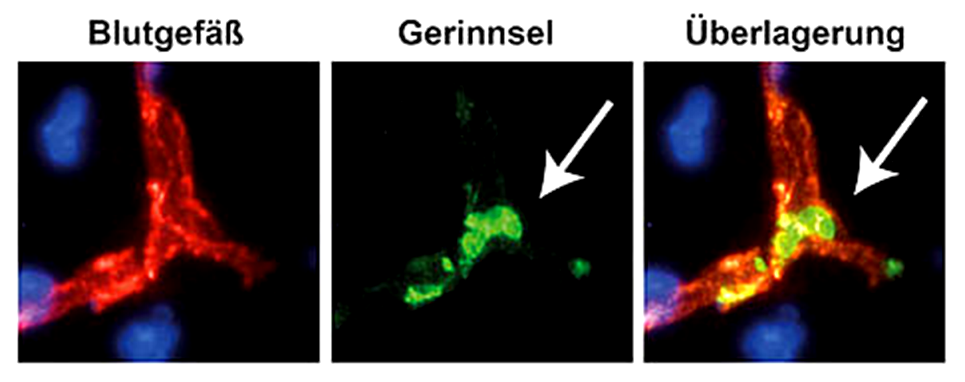
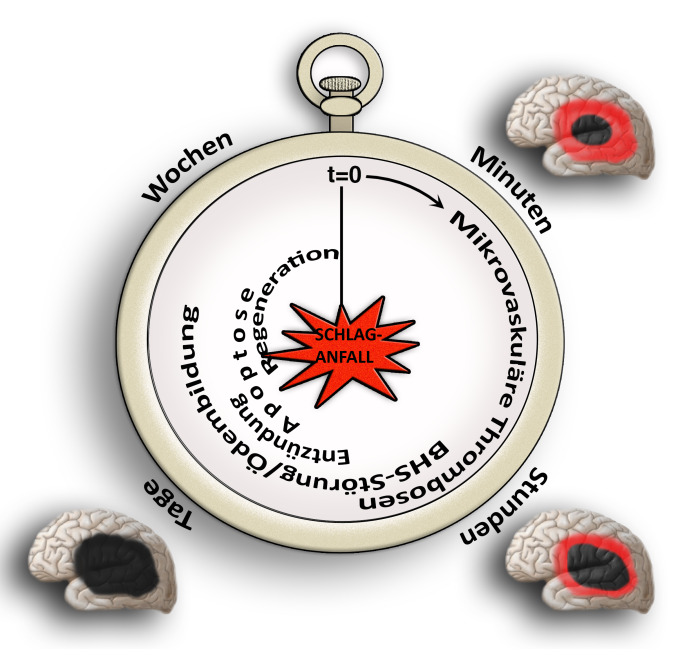
Our research group aims to implement the ischemic stroke as a “thrombo-inflammatory” disease. Thrombo-inflammation means that ischemic stroke is neither a thrombotic, nor an inflammatory disease. The interplay of thrombosis and inflammation is of crucial importance for the secondary stroke pathophysiology. Here, we could identify possible therapeutic targets in the plasmatic blood coagulation system (contact-kinin system), on platelets and specific T cells subtypes.
In addition to therapeutic options in the acute stroke phase, we are also looking into the pathophysiology of the late stroke phase. Here, we test how therapeutic interventions modulating thromboinflammation influence neuronal regeneration, neuronal plasticity and neurological recovery including motor paresis. For enabling the translation of our research findings to the patient situation, we also evaluate therapeutic strategies in stroke pathologies challenged by comorbidities (like old age, arterial hypertension or atherosclerosis).
The heart and the brain are tightly interacting with each other. It is well-known that heart diseases (e.g., atrial fibrillation or cardiac insufficiency) may induce cerebrovascular events via thromboembolic events, while, conversely, ischemic stroke may also induce acute changes of the heart like cardiac arrhythmia. We are evaluating heart – brain interactions in our experimental model systems, examining how thromboinflammatory mechanisms contribute to injury in both tissues. This will help us to define tissue-specific therapeutic targets.
Our research projects are:
- Which is the role of oxidative stress (i.e., formation of free oxygen radicals) in thrombo-inflammatory processes and how can we modulate these mechanisms by new cell specific neuroprotective therapies?
- Which is the role of regulatory T cells (Treg; a subset of CD4+ T cells) in acute and chronic stroke?
- How do new anti-thrombotic agents affect brain injury? Are they safe without increasing bleeding risk?
- Which are the effects of stroke on the cardio-vascular system?
- How do vascular comorbidities (e.g., arterial hypertension, atherosclerosis) influence stroke outcome and how do they alter therapeutic needs?
Publications:
Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gübel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013; 121(4):679-91.
Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 203(3):513-8 (2006).
Güb E, Reymann S, Langhauser F, Schuhmann MK, Kraft P, Thielmann I, Gübel K, Brede M, Homola G, Solymosi L, Stoll G, Geis C, Meuth SG, Nieswandt B, Kleinschnitz C. Blocking of plasma kallikrein ameliorates stroke by reducing thromboinflammation. Ann Neurol. 2015; 77(5):784-803.







