
Mechanisms of neuronal plasticity
Post-stroke, abundant remodeling processes are triggered in the ischemic brain. Nerve fibers, called axons, in healthy brain areas sprout, which contributes to the recovery of lost functions. A significant contribution to functional recovery is attributed to so-called axon collaterals, which re-innervate denervated neurons. By means of histochemical and electrophysiological experiments in cell culture, which are complemented by experiments in brain slices and live animals, the group examines mechanisms underlying neuronal plasticity following stroke.
Our research projects at a glance:
Characterization of axon collateral sprouting and formation of new synaptic contacts following oxygen-glucose deprivation and free radical stress in vitro by histochemical and immunohistochemical techniques (Figure 1), besides others utilizing super-resolution microscopy (e.g., Stimulated Emission Depletion Imaging, STED; Structured Illumination Microscopy, SIM) (Figure 2).
Analysis of reorganisation of neuronal networks following oxygen-glucose deprivation and free radical stress in vitro by electrophysiology (so-called Multi Electrode Arrays, MEA).
Analysis of sprouting of midline-crossing axon collaterals after focal cerebral ischemia in vivo by anterograde and retrograde tract tracing and Golgi-Cox stainin.
Characterization of role of cell cytoskeleton in axonal sprouting, axonal collateral formation, and dendritic and synaptic growth.
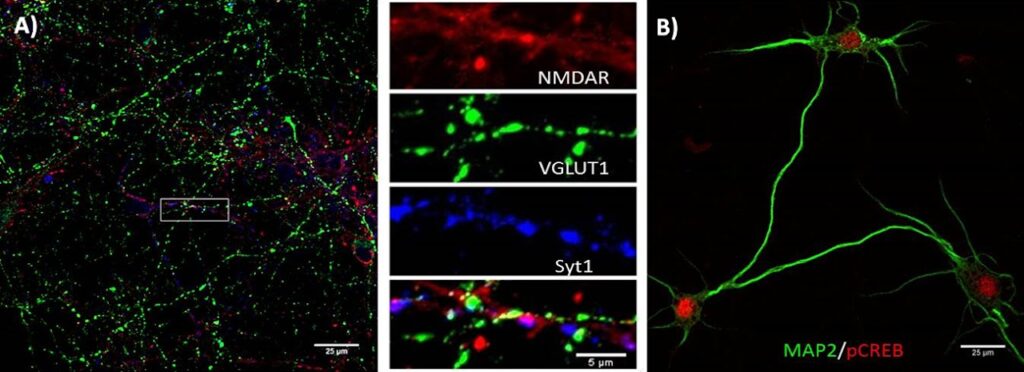
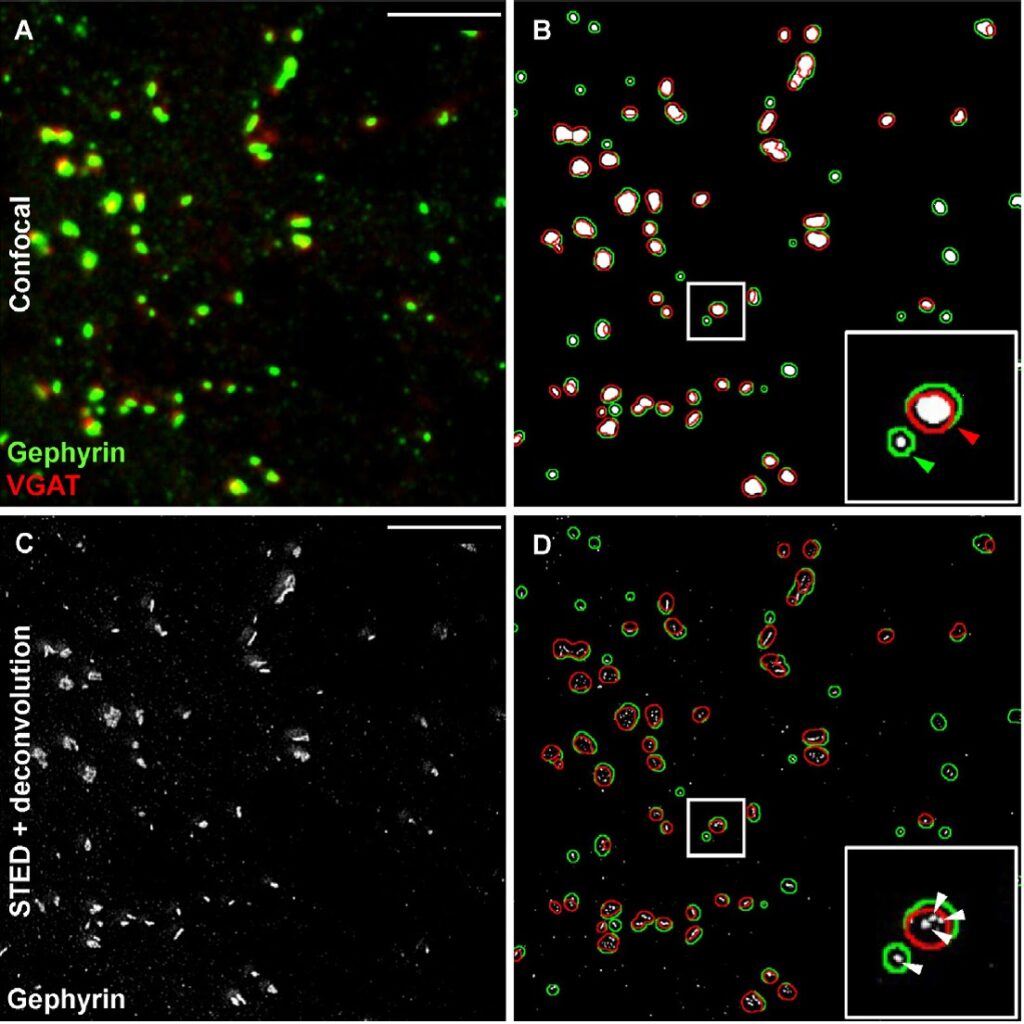
Figure 2. Evaluation of GABA-ergic synaptic contacts by conventional confocal microsopy and superiority of so-called Stimulated Emission Depletion (STED)-microscopy. Synaptic contacts were labeled by (A) confocal immunohistochemistry for the presynaptic protein VGAT (in red) and the postsynaptic protein gephyrin (in green). (B) Co-localisation analysis using conventional confocal microscopy reveals relatively large signal overlaps (shown in red; in green: gephyrin+ areas). (C) Deconvolution using STED microscopy discriminates far smaller protein clusters with a diameter of ~100 nm, which are presumably synapses (D) shows overlay of (B) and (C). Scale bar, 25 µm (from Dzyubenko et al., 2016).
Publications:
Bacigaluppi M, Russo GL, Peruzzotti-Jametti L, Rossi S, Sandrone S, Butti E, De Ceglia R, Bergamaschi A, Motta C, Gallizioli M, Studer V, Colombo E, Farina C, Comi G, Politi LS, Muzio L, Villani C, Invernizzi RW, Hermann DM, Centonze D, Martino G. Neural stem cell transplantation induces stroke recovery by upregulating glutamate transporter GLT-1 in astrocytes. J Neurosci. 2016; 36:10529-10544.
Dzyubenko E, Rozenberg A, Hermann DM, Faissner A. Colocalization of synapse marker proteins evaluated by STED-microscopy reveals patterns of neuronal synapse distribution in vitro. J Neurosci Methods. 2016; 273:149-159.



